
As the shape of the molecule is tetrahedral and Carbon and Chlorine have a difference in their electronegativity. Is CH2Cl2 polar or nonpolar?ĬH2Cl2 Polarity The CH2Cl2 molecule is polar in nature.
#CH2CL2 MOLECULAR GEOMETRY FULL#
Lewis Structures and the Shapes of MoleculesĪnswer and Explanation: C2 H2 Cl2 exists as three isomers: 1,1-dichloroethene, cis-1,2-dichloroethene, and… See full answer below. There are 3 bonds in C2H2 molecule, however there are only two bonds connected to the central atom, C, therefore the hybridization would be sp. How does the hybridization of carbon change as it goes from C2H2 + Cl2 -> C2H2Cl2? The hybridization of carbon changes from sp to sp3. Otherwise, it is polar.ĭichloroethene or dichloroethylene, often abbreviated as DCE, can refer to any one of several isomeric forms of the organochloride with the molecular formula C2H2Cl2: There are three isomers: 1,1-Dichloroethene.
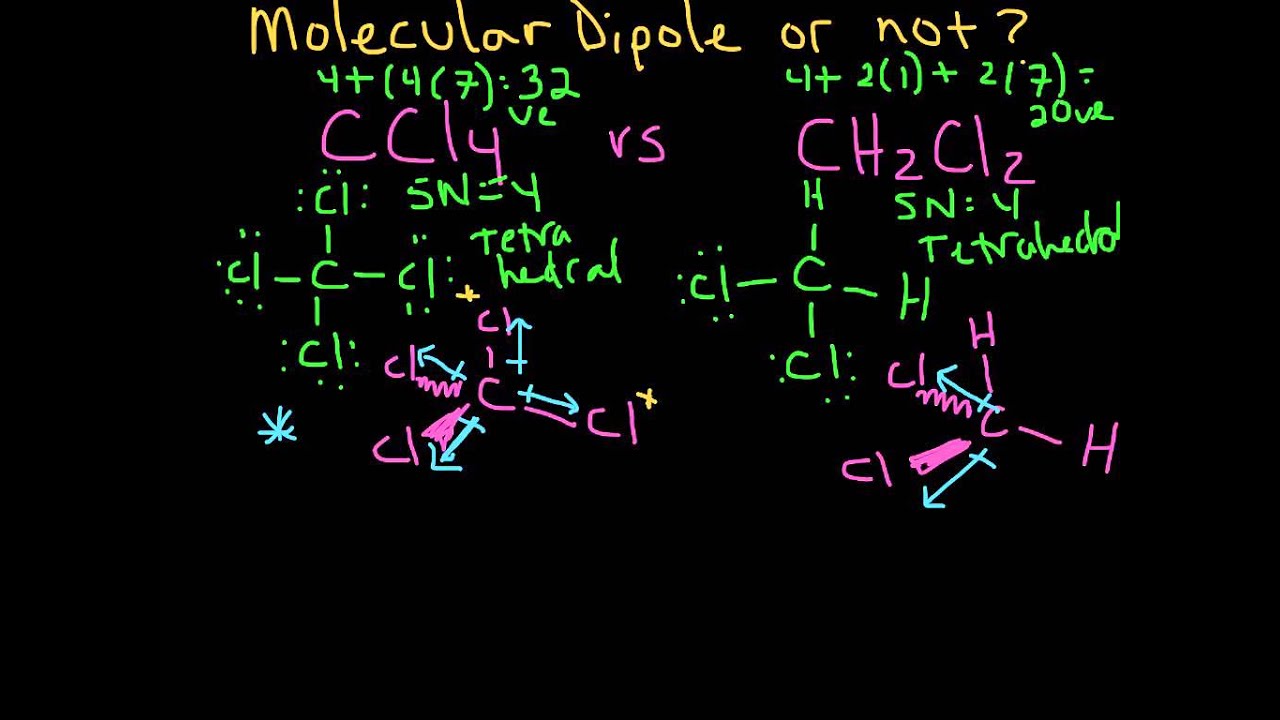
In, the 2 C-Cl bonds create a dipole towards the Cl since Cl is highly electronegative. Re: Why is CH2Cl2 polar and CCl4 is nonpolar? Answer: In the 4 dipoles cancel each other out making the molecule nonpolar. Why do CCl4 and CH2Cl2 have different polarity? The table below shows the Lewis structures and shapes of two molecules.

Is CH2Cl2 asymmetric or symmetrical?Īlthough the bond arrangement around the C atom in CH2Cl2 is symmetrical, the differing polarities of the C–H and C–Cl bonds means the effect of the polar bonds is not cancelled, so the molecule is polar.

Therefore, the molecular geometry for C2H2Cl2 with respect to both carbon central atoms is Trigonal planar. The shape of the compound is a trigonal pyramidal.Īccording to VSEPR theory, the three region of electron density implies that the molecule will be Trigonal planar. What is the molecular shape of CH2Cl2?Īs the hybridization is sp3, the molecular geometry of Dichloromethane becomes tetrahedral. This develops a dipole moment across C-Cl and C-H bonds and the entire molecule results in a net 1.67 D dipole moment. So, Is CH2Cl2 polar or nonpolar? CH2Cl2 is a polar molecule due to its tetrahedral geometrical shape and difference between the electronegativity of Carbon, Hydrogen and Chlorine atoms.


 0 kommentar(er)
0 kommentar(er)
